Phosphorus II - Breaking the law?
In the first of this series of articles, my aim was twofold - to give the history of phosphorus use, from its humble beginnings through to the significant global industry that it is today, and to demonstrate the vast array of functions which phosphorus undertakes within plant and soil systems.
Given the growth of the phosphorus industry, and the sheer volume of scientific literature which supports phosphorus use, one thing is abundantly clear - we cannot do without it. That being said, I believe there to be a number of significant, pressing issues, from sourcing, through the use of the end product and, ultimately, to the environmental fate of the product.
The Law(s):
I'm sure that many of you will be familiar with Liebig's Law of Minimum - this 'law' states that growth is not controlled by the total available nutrient pool, but by the scarcest nutrient. As a basic principle, I like Liebig's Law of Minimum. In field situations, a crop's failure to respond to fertiliser inputs usually points to a limiting nutrient, as per the 'Law of Minimum' - a full spectrum soil test should identify the limiting resource - once this has been identified, a solution can be implemented and healthy growth can recommence.
Despite my liking of the law, I believe it to be severely limited, if not outright inappropriate in certain situations. We work with a very complex system, which compromises not only chemical elements, but also numerous biological processes and physical factors - the Law of Minimum is, in effect, only one dimension of a three dimensional system - it would be erroneous to view it in isolation. With that being said, there is another law; one that I have never seen referred to in our industry, even though I believe it to be far more applicable; 'Shelford's Law of Tolerance', which states: "the abundance or distribution of an organism can be controlled by certain factors (e.g., climate, topographic and biological requirements of plants and animals) where levels of these exceed the maximum or minimum limits of tolerance of that organism".
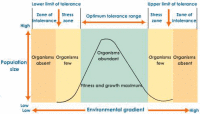
Physical soil properties can also suffer from the effects of too little or too much. For the purposes of this article, however, my focus in respect of the Law of Tolerance is the biological effects of excess phosphorus applications, which brings me on to my next point.
The Science(s):
I think it is fair to say that managing the soil biologically has experienced something of a resurgence in recent years. Utilising biological means for stimulating plant growth isn't a new concept, far from it - in the first of this series of articles, I referenced the fact that Inca colonies utilised human excreta which was collected in pits, to be composted, before being applied to their food crops - the success of this system was fundamentally biological in nature. Successive generations also utilised this practice to great effect. Yes, the success was perhaps more a case of 'luck rather than judgment', but the fact still remains - for centuries, food supplies were, by and large, dependent on the use of human, and other sources of excreta.
Despite the sustained success of the use of excreta, it was not without issue. In 1854, John Snow, a leading English physician, traced the source of a Cholera outbreak to a water pump, which had been installed 3 feet from a leaking cesspit in Soho, London - this discovery led to significant changes in the manner in which waste was dealt with.

Once again, however, history repeats itself - the above theory of plant fertilisation is not without challenge, just as the use of excreta was brought to question in the 19th century. The wider scientific community is slowly acknowledging the importance of biological processes within the soil; something that Liebig's declaration that chemistry would rule agriculture had failed to identify.
With the slow recognition of the importance of biological activity within the soil ecosystem, I would suggest that something of a divide is forming within our industry - two opposing ends of a divisive spectrum, adjoined by a plethora of uncertainty, commercialisation and capitalisation is how I would describe it. On the one hand, there is still a chemical reliance for a large part of the industry, and would still be considered the dominant factor where plant nutrition is concerned; however, some forward thinking individuals and companies have grasped the biological angle, and applied it with aplomb - there are demonstrated cases of these methods being used to great effect - so, what is one to do?
I am not in a position, nor is it my aim, to advise on this issue. It is my aim, however, to present information to you, gained from many hours of research, in this series of articles.

In terms of plant nutrition, phosphorus requirements are commonly asserted via the use of soil testing. There are a number of extractants of differing strengths used for this process, along with various benchmark figures for what would be considered 'Low, Medium or High' values - this lack of clarity is, I believe, the first in a series of flaws within the current system.
It has been suggested that a number of extractants, used in soil testing to determine plant available P, only measure inorganic labile P, therefore completely disregarding the organic pool. Whilst, in certain circumstances, I can see the benefits of testing (identification of limiting nutrients), without a standardised measuring methodology, accurate benchmark figures, and field calibration data, the current soil test protocols, in my opinion, serve as little more than a tool to stimulate fertiliser sales in many cases.
In respect of biological phosphorus acquisition in soils, there are several mechanisms that are employed:
- Various species of Arbscular Mycorrhizal fungi (AMF) have been shown to hydrolyze organic phosphorus and transport it to plant roots, where it is taken up as inorganic phosphorus
- AMF have the ability to modify both the supply and demand relationship for phosphorus acquisition - a technique used particularly where P conditions are limiting, and the demand outstrips potential supply
- Bacterial species, such as Pseudomonas, Azotobacter, Burkholderia, Bacillus and Rhizobium, Actinomycete and Serratia, have been shown to demonstrate variable ability to solubilise P in soils via exudation of citrate, malate, formiate, lactate and succinate, and possibly other dissolution promoting organic substances

Given the above, it would seem wise to foster management techniques that preserve the soil ecosystem as nature intended. It is disappointing to note, however, that many practices that we undertake can disrupt the natural cycles within soils. A number of studies have demonstrated that hyphal growth and hyphal branching induction of mycorrhizal fungi is depressed at high soil P levels. Further studies have also demonstrated this effect - under very high levels of soil P (>50ppm), the plant is often able to acquire a sufficient amount of P so that the mycorrhizal demand is redundant. Mycorrhizal development in roots is often reduced in such circumstances.
Conversely, under what would normally be considered low P levels (5-20ppm), P acquisition by AMF can result in a net growth benefit to the plant. These studies would all confirm that, whilst P is required for plant growth, excessive applications can suppress microbial acquisition.
My issue with excess P applications is not limited to suppression of microbially acquired P mechanisms however, so I ask, if Mycorrhizal acquisition of P is suppressed, would it be safe to assume that the AMF become redundant? If this is the case, what happens to the remainder of the processes that AMF undertake, such as disease suppression, soil aggregation, enhanced water uptake etc?
In addition to the above, research also suggests that, on average, 30-65 percent of the total soil P is in organic forms - these compounds are mainly inositol phosphates, nucleic acids and phospholipids. This leads me to pose the following questions - given that a number of extractants only measure the inorganic phosphorus portion, if a soil test result demonstrates a 'low' reading, is the soil actually low in phosphorus, or could it be that the organic pool is in plentiful supply and, by responding to a low reading via the application of further fertiliser to correct levels, are we acting counter productively by further suppressing the various microbial acquisition mechanisms?

When Liebig proclaimed that chemistry would revolutionise agriculture, I'm sure he envisaged only positives. With the benefit of hindsight, however, having noted one scientific paper after another report on the world's declining soil quality, I would have to question Liebig's claims. I would summarise my own feelings in the words of Lady Eve Balfour, from her seminal book; The Living Soil, 1943:
"When water borne sewerage was introduced to our cities, the capital of the soil - its fertility - which is removed from it year by year in the form of crops and livestock - no longer found its way back to the land in the form of waste products of the community, but was poured into the sea, or otherwise destroyed".
The environmental(s):
Environmental concerns run abound when discussing the subject of phosphorus - certainly too many to discuss in one article. For this reason, I will focus on what I consider to be the two main issues:
Phosphogypsum - a by-product of the wet process production of phosphoric acid. For every metric tonne of phosphoric acid produced, approximately five tonnes of Phosphogypsum on a dry weight basis is generated. It is important to note however, that this by-product is not the gypsum which many of us use as a soil conditioner. Whilst many characteristics are similar, Phosphogypsum is known to contain a number of potentially toxic elements. It primarily consists of calcium sulphate dihydrate, with small amounts of silica and phosphate rock. The toxic elements contained include, but are not limited to, arsenic, cadmium, chromium, mercury and fluoride. In addition to the aforementioned elements, appreciable quantities of radioactive materials, in the form of radon, thoron and uranium, are also present.
Given the sheer quantity of Phosphogypsum that is generated, it poses something of a problem for the fertiliser industry - put simply, what is it to do with such a mass of a by-product which carries such environmental risks? Re-use is a difficult proposition, given strict regulations regarding its use - there are limited uses in agriculture and road construction, where the radiation levels are proven to be low; however, in reality, this only represents a very small portion of the product. Current surveys of global Phosphogypsum suggest that 14% is re-used, 58% is stored in gyp-stacks, and the remaining 28% is discharged to inland watercourses and the sea.
- The acidic nature of the Phosphogypsum keeps the toxic elements in a leachable form
- Transport of the toxic elements can pollute the nearby surroundings - the main vectors for this are wind and water erosion, infiltration and leaching to groundwater
- Erosion of constructed slopes, berms and dikes can lead to surface runoff
Whilst it is clear that various countries have imposed more stringent safety measures over recent years, to ensure that none of the above risk factors become actual occurrences, it should also be noted that they are far from failsafe. There is a history of stack failures, such as dike breaches, overflowing stacks and sinkholes below stacks - all of which have led to varying degrees of environmental contamination.
The Baltic Sea is a much discussed topic where contamination from gyp-stacks is concerned. The most famous cases in recent years are the Kingisepp plant in Russia, and both the Gdansk and Police plants in Poland - extremely high phosphorus concentrations have been found in the Dead Vistula, a waterway that runs adjacent to the Gdansk plant. In respect of the Police plant, elevated levels of phosphorus concentrations downstream indicate leaching from the gyp-stacks. In Kingesipp, Russia, a significant leakage from a gyp-stack into the Luga River was reported in the Spring of 2012. All of the above waterways discharge directly into the Baltic Sea.
In respect of direct disposal into inland watercourses, and the sea, this could be considered as another questionable practice, and one that has come in for severe criticism from a number of quarters. As with the use of gyp-stacks, there are a number of guidelines in place; however, independent assessments of the levels of toxicity of a number of the inland waterways would suggest that existing control measures are insufficient.
In France, it has been reported that 3 million tonnes (Mt) have been dumped in the Seine estuary to date - significantly raised levels of radionuclides have been reported in mussels collected from the area. In the UK, large amounts of Phosphogypsum were dumped into the Solway Firth between 1954 and 1992 - thankfully, this practice ceased in '92. There is also a history of a similar trend in the Netherlands, with the Rhine estuary being a point source for Phosphogypsum disposal ... and so, on goes the story.

Florida State regulators' "worst nightmare" happened in June 1994 when a cavernous hole, 106ft. wide by 185ft. deep, opened in the centre of an IMC-Agrico waste stack near Mulberry, like a scene out of Jules Verne's Journey to the Centre of the Earth. The sinkhole released 20.8 million pounds of liquid phosphoric acid into the ground below. The company was able to clean up the spill before it harmed the drinking water supply, regulators say
In a particularly troublesome and somewhat poignant series of events which combine both methods of disposal, I would point to the much maligned Piney Point phosphate plant, located in Tampa, Florida. To give a brief history of this controversy plagued plant, reports from the 1960s, with suggestions of the death of many local fish stocks and cattle poisoning, were rife. The 1970s and 80s were marred by numerous breaches of EPA and local government policies, which resulted in repeated fines. 1989 saw the evacuation of hundreds of residences in the local area, courtesy of a large sulfuric acid spill - this was to be followed by the death of three workers in 1991. The plant has changed hands on numerous occasions, and bankruptcy has been a recurring theme.
My concerns, however, are limited to one particular instance. Following the closure of the plant by former owners, concerns were raised repeatedly regarding the potential spillage of approximately 500 million tonnes of toxic wastewater from the gyp-stacks, into the nearby community and, ultimately, Tampa Bay. The potential cause of this spillage? High levels of rainfall. That is correct, high levels of rainfall; the would be trigger to what could only be described as a catastrophic toxic waste spill - this in Tampa, Florida - an area that is known for its excessively high rainfall and repeated tropical storms.
The answer to this dilemma? Take 248 Mt of the toxic waste out into the Gulf of Mexico in barges and dump it 40 miles off the coast. The cost of this was purported to be between $15,000,000 - $37,500,000… of tax payers money. For all of the research that I have undertaken in an effort to enable me to give a complete overview of this troublesome sector, each time I read of this particular occurrence, I feel compelled to take a moment to pause - a very sad indictment indeed.
It is with a degree of concern that I write of the above, and even moreso when I highlight the fact that, whilst Piney Point is an extreme case, it is not an isolated one. There are many more such stories from plants in Florida, Mississippi, Spain and further afield. Whilst I appreciate that regulatory bodies are implementing further control measures, I would have to point to the basic fundamental principles of risk management - the first, and most successful of which is to remove the risk.

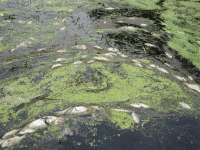
Eutrophication - Another significant environmental threat is that of eutrophication - this ecological response is fuelled by nutrient enrichment of inland and coastal waterways. Increased algal biomass is the net result of this nutrient enrichment, and leads to anoxic conditions, thereby leading to the death of several marine species, including important fish and invertebrate stocks. Furthermore, algal blooms and invasive marine plant species become rife, and reductions in species biodiversity are commonly referred to.
Phosphorus and nitrogen are the two main contributory factors to this condition; however, for the purposes of this article, I will solely focus on the contribution of phosphorus. I am sure that many of you will have seen images of algal blooms in waterways - they are not an uncommon sight, but I ask, how many stop and think of the cause? How many stop and consider what we contribute to this issue?
As nutrient enrichment is the prime driver of this ecosystem response, I would suggest that Liebig's Law of Minimum takes centre stage here. Phosphorus is widely acknowledged as the primary limiting factor for eutrophocation - put simply, if we limit the amount of phosphate which finds its way to our inland and coastal waters, we limit eutrophication.
The two main vectors for phosphorus movement into waterways are run off and leaching. There are several mechanisms that can be employed to reduce surface run off - the most successful of which is to simply ensure adequate levels of ground cover. Leaching is another, perhaps more significant issue where our industry is concerned - most will happily state that phosphate is immobile in soils - I will stick my neck out and argue this point - phosphorus is mobile in soil, and does leach, particularly where excess levels above plant requirements are found.
With high specification drainage schemes commonplace in many a venue now, along with high levels of phosphorus fertilisation, many of us are unwittingly contributing to the point source of phosphates entering our waterways - it is time that our industry acknowledged this fact, and I will add in that this should be the case for all known environmental pollutants, as I state that alternative products, and alternative methods are key points for our industry to reduce its environmental burden.
To summarise my feelings from the points raised in this, the second in this series of articles, I think it is clear, from the passage of the information contained in this article, that the phosphate rock processing industry is not without issue. It is a fact that overuse of phosphate fertilisers suppresses the soils natural phosphorus acquisition mechanisms, and it may come as a surprise to many to note that this rule applies to organic sources as well as mineral - quantity is the driver of biological shutdown, not source material.
With that said, the additional contaminants contained within mineral sources can accumulate in soils and lead to plant and biological phytotoxicity, as per the rules of the Law of Tolerance. Purely from an agronomic perspective, it is my belief that first and foremost, it is incumbent upon us to protect the natural processes of the soils which we work - the way that this is achieved is twofold.
Firstly, there is a fundamental lack of reference to suitable and sufficient complex carbon sources in this industry - complex carbon sources are vital to ensuring that microbial biota have the foundation to proliferate from. Secondly, the overuse of a number of products simply must come to an end. I have demonstrated the effects of 'enrichment' of watercourses in the section regarding eutrophication - it is my belief that the negative effects of this enrichment are not limited to waterways, and I believe that soils are equally as susceptible to such devastation - we only have to look at many of the turf diseases and disorders that prevail in our industry nowadays to see that many of the problems are of our own making. A vicious circle has been created, and it will never be solved by adding to it.
The environmental issues are beyond dispute - the question therefore is, are we willing to acknowledge the part that we play in these issues, and seek alternative methods, or are we to bury our heads in our rootzones whilst we strive for the perfect playing surface, irrespective of the cost?

In the next, and final piece in this series of articles, I will raise the issue of the long term sourcing of phosphorus, and will offer my thoughts on what alternative products are available now, and perhaps in the future, in addition to what alternative management strategies could be deployed to aid with maximising the biological cycling of phosphorus in soils.
